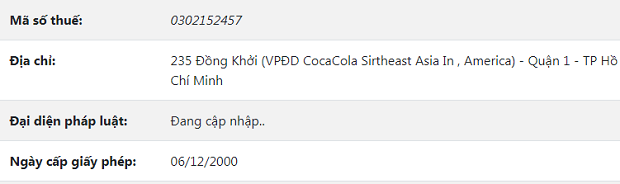
Job Summary:
- Maintain and lead SC management system and ensure compliance based on fulfillment in implementing all requirements of KORE/ ATR/MECQ/McD, Government, ISO 9001 and FSSC 22000, customers at plant and Copackers.
- Play as Master Trainer, coordinator and auditor of system at plant.
- Play as an administrator of e-paperless system at plant.
Key Responsibilities:
1. Manage & Control SC Managment system of Region (Plant and Co-packers)
- Maintain SC management system activities at regional plant and copackers about KORE/ATR/MECQC/McD/[protected info] Quality, food safety compliance, local law compliance, ISO 9001 & FSSC 22K (Management review, Tracebility, MOC recall, HACCP plan, ...), GMP audit and other audits.
- Contribute to build SC management system with QA-CO and be in charge of deploying any new requirement/changes in SC Management System at regional plant and Co-packers.
- Create, standadize, review and update SC documents system and internal SOPs at region level: Kore, ATR, Local laws, 3rd party requirement, FSSC 22000 and ISO9001 with one system.
- Be in charge of system capability development for plant team such as the awareness and internal auditors training (ISO 9K, FSSC22K, GMP, ISO 45k, ISO 14k), Food Safety awareness/certificate for personnel...
- Follow-up full test plan and make sure full test result are complying with local law.
- Lead quality activities about Food Safety Culture at plant (Pest control, HACCP Verification/ Validation)
2. Audit and Compliance at Plant and Co-packers:
- Participate, review & set up plan/ activities for internal audit and contribute to all external audits such as ISO9001, FSSC22000, GMP, SWAT, Food Safety & Quality audit from authorities, GAO, ...
- Mainly routine audit (as weekly/ monthly/ quarterly audit program) at plant/ Copacker for evaluation and implementation of SOPs and Systems needed to comply with regulatory requirements including partial audit, GMP audit, SWAT.
- Review findings and follow-up SC CAP closing for all CAPs (audits, inhouse, complaint,...)
- Tracking EOSH CAP status by follow up with EOSH Manager
- GMP leader: lead GMP audit activities, track, propose and follow-up actions to improve the status of Plant's GMP.
- Follow up the status of certificates: Food safety certificates from Government, ISO 9001:2015; FSSC 22000
- Set up and maintain pest control system at plant
- Lead activities on water testing and send report to Authorities as required (in Da Nang only)
- Review and update new local law, KORE/ ATR when be informed from MSC-CO to take action if needed
3. Reporting & best practice learning:
- Weekly, monthly QA report: customer/ consumer complaint trend, quality weekly highlight, CAP completion, GMP score,...
- Monthly Swire/ SC/Best fresh, market sample, pest control, full test review or as required
- Update status of Management review status, GAO status, and CAPs
- Seek for QSE best practice, communicate and share to apply at plant
4. E-paperless administrator
- Work with IT, Head Office to solve quickly for all issues relating to e-paperless
- Follow-up all workflows at regional plant completed in e-paperless system
- Provide Training to Users & curricula development to ensure users at plant are familiar to system
5. Other tasks are assigned by Direct Line Manager or
QA Manager